Correlation Between the Clinicopathological Features of Papillary Thyroid Carcinoma Complicated with Hashimoto's Thyroiditis, BRAF V600E Gene Mutation, and RET Gene Rearrangement
By Min Xie1, Zeng Xiang Xu2, Min Dai1, Yong Yun Zhu3Affiliations
doi: 10.29271/jcpsp.2024.04.445ABSTRACT
Objective: To analyse the expression of BRAF V600E protein and RET gene rearrangement in papillary thyroid carcinoma (PTC) combined with Hashimoto's thyroiditis (HT) and to explore its clinical and pathological significance.
Study Design: Observational study.
Place and Duration of the Study: Department of Pathology, East China Normal University (Wuhu No. 2 People’s Hospital), Wuhu, China, from January 2019 to July 2022.
Methodology: The study population of 150 patients who underwent central lymph node dissection. They were divided into two groups: the PTC group (76/150, 50.7%) and the PTC with HC group (74/150, 49.3%). The expression of BRAF V600E protein was detected using immunohistochemistry, and the RET gene rearrangement status was detected using fluorescence in situ hybridisation. The detection results and clinical pathological characteristics were statistically analysed.
Results: Compared with the PTC group, the prevalence rate of female PTC in HT group was significantly higher than that of the male group, the rate of lymph node metastasis was lower, and the proportion of tumour diameter ≤ 1cm was higher (p < 0.05). However, no significant difference in patient age and multifocality was found between the two groups (p > 0.05). The BRAF V600E positive rate in the PTC combined with HT group (48.6%) was lower than in the PTC group (73.7%), and the RET gene rearrangement positive rate was higher than in the PTC group (p < 0.05). The expression of BRAF V600E protein in PTC combined with HT is correlated with multifocality (p < 0.05), and there is a correlation between RET gene rearrangement and the gender of the patient in the PTC group (p < 0.05).
Conclusion: There is a lower rate of BRAF V600E protein positivity in PTC combined with HT patients, as well as a higher rate of RET gene rearrangements positive in PTC combined with HT patients. There is a correlation between multifocality and BRAF V600E protein expression.
Key Words: Papillary thyroid carcinoma, Hashimoto's thyroiditis, BRAF V600E protein, RET gene rearrangement.
INTRODUCTION
Thyroid cancer (TC) is the most common endocrine malignancy, with papillary thyroid carcinoma (PTC) being the most common form, accounting for 80% of all diagnosed TCs.1 Hashimoto's thyroiditis (HT) is a chronic inflammation of the thyroid gland, first described over a century ago, and is now considered the most common autoimmune disease.2,3 The incidence of PTC coexisting with HT is increasing rapidly, with a rising trend year on year.4
It has also been reported that the co-existence of these two diseases ranges from 10 to 58%,5 and the correlation between the two has become a research hotspot.6-8 Certain genetic alterations of PTC were identified to take part in the pathogenetic process: BRAF and RAS point mutations and RET/PTC oncogene rearrangements.9 RET/PTC rearrangement is a common genetic abnormality in PTC, which may contribute to the early development of PTC. On the contrary, BRAF mutations are hardly expressed in HT but are relatively more common in PTC. Therefore, this study aimed to detect the expression of BRAF V600E protein and RET gene rearrangement in PTC coexisted with HT, and to analyse the possible clinicopathological relationship between HT and PTC, to provide a theoretical foundation for the study of its pathogenesis, progression and prognosis.
METHODOLOGY
After approval by the Ethical Committee at Wuhu No. 2 People’s Hospital, a retrospective analytical analysis of the medical records of a series of 150 patients was conducted. The patients were selected among 150 undergone hemilobar/total thyroidectomy and therapeutic or preventive neck dissection in the period from January 2019 to July 2022. All patients with complete clinicopathological data were considered eligible for the study. Only patients with PTC were included, whereas all other types of thyroid cancer were excluded. PTC patients with a history of radiation exposure were also excluded. The pathological diagnoses were confirmed by two experienced diagnostic pathologists according to World Health Organisation standards. The patients were divided into two different groups. The first group consisted of 74 patients for PTC; the second group consisted of 76 patients for PTC with HT. The clinicopathological parameters, include age (< 55 or ≥ 55), gender (male or female), tumour size (≤ 1cm or > 1cm), multifocality, vascular invasion, and central lymph node metastases (CLNM).
The primary reagents are the Ventana IHC kit (purchased from Roche Diagnostics), which contains a mouse anti-human VE1 monoclonal antibody, the OptiView Amplification Kit, the OptiView IHC Assay Kit, and a rabbit monoclonal negative QC antibody and QC slides, and the RET (10q11) gene breakage probe kit (purchased from Anbipin, Guangzhou, China).
One hundred and fifty cases of PTC paraffin tissues were sectioned at 4 μm thickness and placed in a constant temperature incubator at 60℃ overnight. Staining was performed on a Benchmark XT automated immunohistochemistry stainer (Ventana, USA) and the staining procedure was described in the manufacturer's kit. Sections from known positive tissues were used as positive controls, and PBS instead of primary antibody was used as a negative control.
Detection of RET gene expression by fluorescence in situ hybridisation is performed according to the kit steps, including slide pre-treatment, simultaneous denaturation/hybridisation of sample and probe (light-avoidance operation), post-hybridisation washing, and re-staining (light-avoidance operation).
BRAF V600E antibody staining was observed in the cytoplasm, and the result was determined by referring to both the staining intensity (0-3+) and the positive percentage (0-100%). When the percentage of positive was ≥ 20% and the staining intensity was ≥ 2+, it was defined as positive. FISH interpretation of RET gene, negative: 2F (two fusion), indicating no breakage; positive: 1R1G1F (one red, one green, one fusion), suggesting breakage, 1G1F (one green fusion), suggesting deletion of the 5 'end (red) of RET gene, and tyrosine kinase region (green).
All statistical data analyses were run on the SPSS 25.0 (IBM, NY, USA). The Chi-squared test was used to compare frequencies with percentages of qualitative variables. Analyses of multivariate logistic regression were conducted to identify the associated risk factors for PTC complicated with HT. To determine the statistical significance of these associations, a p-value of <0.05 was used.
RESULTS
Among the 150 cases of PTC, 74 cases were in the group of PTC with HT and 76 cases were in the group of simple PTC without HT. PTC patients < 55 years old group accounted for 73.7% (56/76); females accounted for 78.9% (60/76); tumour diameter ≤ 1cm accounted for 56.6% (43/76); multiple tumours accounted for 40.8% (31/76), and central lymph node metastasis accounted for 51.3% (39/76). Compared with the simple PTC group, the occurrence rate was higher in the PTC with HT group (93.2% vs. 73.7%, p < 0.05). The proportion of tumour diameter ≤ 1cm is higher (75.7% vs. 56.6% p < 0.05), and the rate of lymph node metastasis was lower (28.4% vs. 51.3%, p < 0. 05). It was not related to age and multiple tumours (p >0. 05, Table I).
Positive staining for BRAF V600E protein was cytoplasmic (Figure 1). PTC+HT group had lower BRAFV600E positivity than the PTC group (48.6 vs. 73.7%), and the difference was found to be statistically significant (p < 0. 05, Table I).
Positive RET gene rearrangements were found in 28.7% of PTC. The PTC+HT group had a higher positive rate than the PTC sample (37.8% vs.19.7%), and the difference was statistically significant (p < 0.05, Table I).
In 74 cases of PTC with HT, the positive rate of BRAF V600E protein was 48.6%, and the positive rate of RET rearrangement was 37.8%. Statistical analysis showed that BRAF V600E protein expression had no correlation with age, gender, tumour size, and lymph node metastasis, but was associated with multiple tumours (p < 0.05), while RET gene rearrangement was not correlated with age, gender, tumour size, tumour recurrence, and lymph node metastasis. BRAF V600E protein was more frequently detected in 76 cases of PTC with HT, but there was no correlation with age, gender, tumour size, tumour recurrence and lymph node metastasis (p > 0.05), and the rearrangement of RET gene had no correlation with age, tumour size, tumour recurrence and lymph node metastasis, but related to gender (p < 0.05, Table II).
Based on multivariate logistic regression analysis, the risk factors associated with HT in PTC included age, gender, tumour size, multiple tumours, lymph node metastasis, and expression of the BRAF V600E protein and RET gene rearrangement. The results showed that HT was positively correlated with the age of onset and positive RET gene rearrangement. It was negatively correlated with the positive expression of BRAF V600E protein, lymph node metastasis and the tumour diameter. It was not related to the gender and frequent occurrence of tumours (Table III).
Table I: Relationship between HT and clinicopathological features in PTC [n (%)].
|
Characteristics |
n |
PTC + HT |
PTC |
p |
|
Age (year) |
|
|
|
|
|
<55 |
113 (75.3%) |
57 (77%) |
56 (73.7%) |
0.635 |
|
≥55 |
37 (24.7%) |
17 (23%) |
20 (26.3%) |
- |
|
Gender |
- |
- |
- |
|
|
Male |
21 (14%) |
5 (6.8%) |
16 (21.1%) |
<0.05 0.012 |
|
Female |
129 (86%) |
69 (93.2%) |
60 (78.9%) |
- |
|
Tumour size (cm) |
- |
- |
- |
|
|
≤1 |
99 (66%) |
56 (75.7%) |
43 (56.6%) |
<0.05 0.014 |
|
>1 |
51 (34%) |
18 (24.3%) |
33 (43.4%) |
- |
|
Multifocal |
- |
- |
- |
|
|
Yes |
64 (42.7%) |
33 (44.6%) |
31 (40.8%) |
0.638 |
|
No |
86 (57.3%) |
41 (55.4%) |
45 (59.2%) |
- |
|
CLNM |
- |
- |
- |
|
|
Yes |
60 (40.0%) |
21 (28.4%) |
39 (51.3%) |
<0.05 0.004 |
|
No |
90 (60.0%) |
53 (71.6%) |
37 (48.7%) |
- |
|
BRAF V600E mutant protein expression |
- |
- |
- |
|
|
Positive |
92 (61.3%) |
36 (48.6%) |
56 (73.7%) |
<0.05 0.002 |
|
Negative |
58 (38.7%) |
38 (51.4%) |
20 (26.3%) |
- |
|
RET/PTC Rearrangement |
- |
- |
- |
|
|
Positive |
43 (28.7%) |
28 (37.8%) |
15 (19.7%) |
<0.05 0.014 |
|
Negative |
107 (71.3%) |
46 (62.2%) |
61 (80.3%) |
- |
|
Chi-square test; CLNM, Central lymph node metastases; p < 0.05, Statistically significant; n, Number of patients. |
||||
Table II: Association of BRAF V600E protein and RET Rearrangement with clinicopathology different variables of thyroid cancer patients.
|
Characteristics |
PTC+HT |
PTC |
||||||||||
|
BRAF V600E mutant protein expression |
p |
RET/PTC Rearrangement |
p |
BRAF V600E mutant protein expression |
p |
RET/PTC Rearrangement |
p |
|||||
|
+ |
- |
|
+ |
- |
|
+ |
- |
|
+ |
- |
|
|
|
Age (years) |
|
|||||||||||
|
<55 |
30 (83.3%) |
27 (71.1%) |
|
21 (75%) |
36 (78.3%) |
|
43 (76.8%) |
14 (70.0%) |
|
16 (100.0%) |
41 (68.3%) |
|
|
≥55 |
6 (16.7%) |
11 (28.9%) |
7 (25%) |
10 (21.7%) |
13 (23.2%) |
6 (30.0%) |
0 (0%) |
19 (31.7%) |
||||
|
Gender |
|
|||||||||||
|
Male |
2 (5.6%) |
3 (7.9%) |
|
1 (3.6%) |
4 (8.7%) |
|
14 (25.0%) |
2 (10.0%) |
|
0 (0%) |
16 (26.7%) |
<0.05 |
|
Female |
34 (94.4%) |
35 (92.1) |
27 (96.4%) |
42 (91.3%) |
42 (75.0%) |
18 (90.0%) |
16 (100.0%) |
44 (73.3%) |
||||
|
Tumour size (cm) |
|
|||||||||||
|
≤1 |
30 (83.3%) |
26 (68.4%) |
|
22 (78.6%) |
34 (73.9%) |
|
32 (57.1%) |
11 (55.0%) |
|
11 (68.8%) |
32 (53.3%) |
|
|
>1 |
6 (16.7%) |
12 (31.6%) |
6 (21.4%) |
12 (26.1%) |
24 (42.9%) |
9 (45.0%) |
5 (31.2) |
28 (46.7%) |
||||
|
Multifocal |
|
|||||||||||
|
Yes |
9 (25.0%) |
24 (63.2%) |
<0.05 |
9 (32.1%) |
24 (52.2%) |
|
21 (37.5%) |
10 (50.0%) |
|
6 (37.5%) |
25 (41.7%) |
|
|
No |
27 (75.0%) |
14 (36.8%) |
19 (67.9%) |
22 (47.8%) |
35 (62.5%) |
10 (50.0%) |
10 (62.5%) |
35 (58.3%) |
||||
|
CLNM |
|
|||||||||||
|
Yes |
12 (33.3%) |
9 (23.7%) |
|
7 (25.0%) |
14 (30.4%) |
|
31 (55.4%) |
8 (40.0%) |
|
6 (37.5%) |
33 (55.0%) |
|
|
No |
24 (66.7%) |
29 (76.3%) |
|
21 (75.0%) |
32 (69.6%) |
25 (44.6%) |
12 (60.0%) |
10 (62.5%) |
27 (45.0%) |
|||
|
Chi-square test; CLNM, Central lymph node metastases; p < 0.05, Statistically significant; n, Number of patients. |
||||||||||||
Table III: Multivariate Logistic regression analysis of risk factors PTC complicated with HT.
|
Variables |
OR (95%CI) |
p-value |
|
Age (years) (<55 vs. ≥55) |
3.68 (1.272-10.644) |
0.016 |
|
Gender (male vs. female) |
0.835 (0.397-1.758) |
0.635 |
|
Tumour size (cm) (≤1 vs. >1) |
0.419 (0.208-0.842 |
0.015 |
|
Multifocal (Yes vs. No) |
1.168 (0.611-2.233) |
0.638 |
|
CLNM (Yes vs. No) |
0.376 (0.191-0.739) |
0.005 |
|
BRAF V600E mutant protein expression |
0.338 (0.171-0.671) |
0.002 |
|
RET/PTC Rearrangement |
2.475 (1.187-5.161) |
0.016 |
DISCUSSION
With the improvement of medical technology and level, the detection rate of papillary thyroid cancer and Hashimoto's thyroiditis gradually increased, and the incidence of their combination also showed an increasing trend. It has been reported that shows that the ratio of PTC merging HT is 23.2 -30.2%, and the proportion of women is 72.5 -88. 3%.10,11 The result of this study is consistent with it, the ratio of PTC combined with HT is 49.3%, most of them are females, accounting for 86%, which indicates that PTC and HT occur at the same time, and most of them are female.
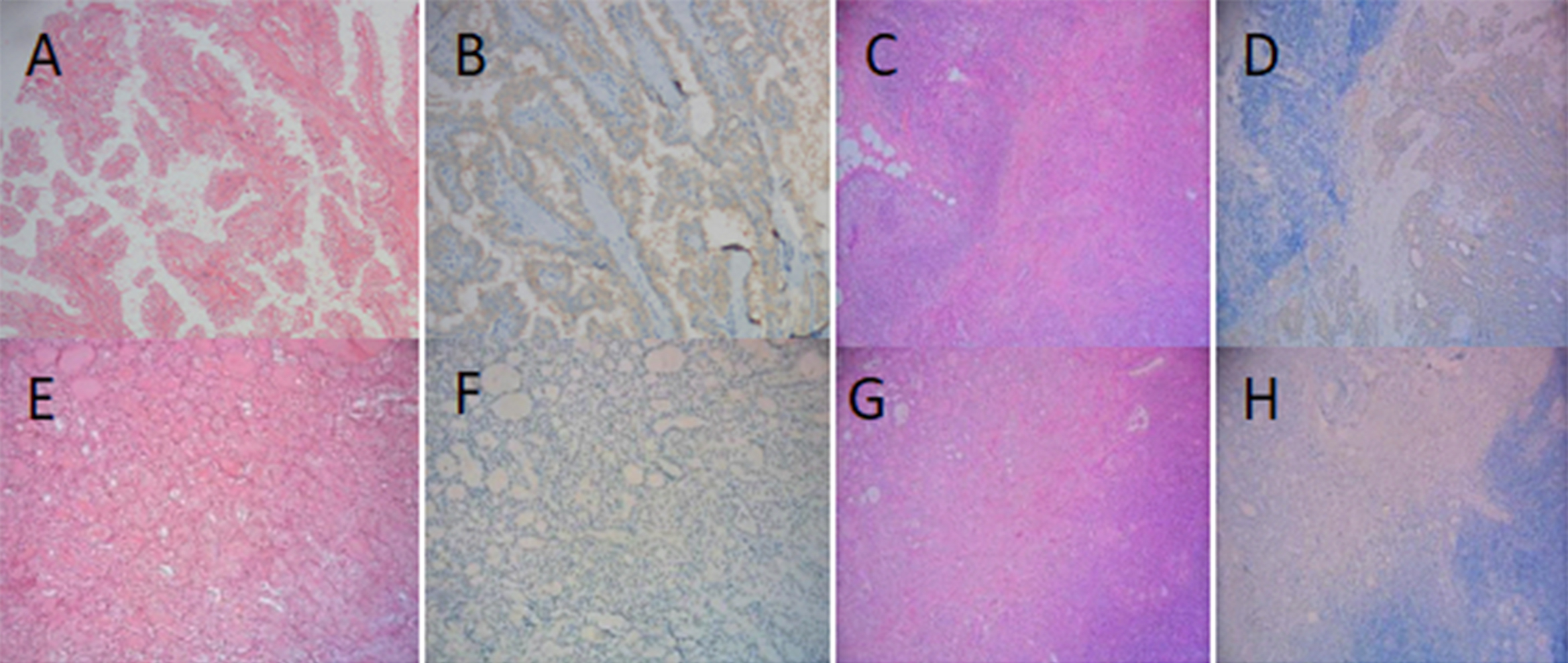 Figure 1: Expression of BRAF V600E protein in two groups. (A) PTC (HE staining×200); (B) PTC BRAF V600E positive (IHC×200); (C) PTC +HT (HE staining ×100); (D) PTC + HT BRAF V600E positive (IHC×100); (E) PTC (HE staining ×200); (F) PTC BRAF V600E negative (IHC×200) (G) PTC+HT (HE staining×100); (H) PTC+HT BRAF V600E negative (IHC×100).
Figure 1: Expression of BRAF V600E protein in two groups. (A) PTC (HE staining×200); (B) PTC BRAF V600E positive (IHC×200); (C) PTC +HT (HE staining ×100); (D) PTC + HT BRAF V600E positive (IHC×100); (E) PTC (HE staining ×200); (F) PTC BRAF V600E negative (IHC×200) (G) PTC+HT (HE staining×100); (H) PTC+HT BRAF V600E negative (IHC×100).
In order to evaluate the effect of HT on the clinicopatho-logical features of PTC, 150 cases of PTC were collected and divided into two groups according to the presence of HT. Several trials showed that HT has a protective role in PTC progression, with fewer lymph nodes metastasising in PTC patients treated with combined HT.12 In this study, the lymph node metastasis rate of the PTC with HT group was lower and the proportion of tumours ≤ 1cm in diameter was higher compared with the simple PTC group. However, there was no significant difference in age and tumour frequency.
BRAF gene mutations are particularly common in PTC, with mutation rates ranging from 29% to 83%.13,14 The prevalence of the BRAF V600E mutation in the Chinese population was significantly higher than in the Western population. Two studies based on Chinese patients reported a prevalence of 83.7 and 75.4%, respectively.15,16 At present, the direct sequencing method and Real-time PCR method are widely used in BRAF gene mutation detection platforms. These two methods have high detection accuracy, but the operation is more complex and the detection cycle is longer. Therefore, it is important to choose the appropriate method to detect BRAF gene mutation. In this study, full-automatic immuno-histochemical method combined with BRAF V600E mutation-specific antibody VE1 to detect BRAF mutation, and the consistency with Sanger sequencing method is more than 95%. The OptiView amplification kit detection system of Ventana IHC adopts a special non-endogenous semi-antigen system, which makes the detection more sensitive and specific. Even if there are few tumour samples, they can be detected, are easy to interpret, and have the advantages of low cost and high efficiency, so it is more suitable as a primary screening method and is gradually becoming a common method for BRAF gene mutation detection. In this study, the positive rate of BRAF V600E protein in the PTC was 61.3%. The positive rate of BRAF V600E protein in PTC plus HT group was lower than that in the simple PTC group (48.6 vs.73.7%), and the difference was statistically significant. HT was negatively correlated with the positive expression of BRAF V600E protein.
RET gene rearrangement is called PTC carcinogen, which is a unique molecular marker of PTC. The incidence of RET rearrangement in PTC is about 2.5 to 73%.17,18 Studies have shown that RET rearrangement is related to gender, age, tumour size, TNM stage, multiple lesions, extracapsular invasion, lymph node metastasis, and distant metastasis in patients with PTC.19 In this study, there were 28 cases of RET/PTC rearrangement in cancer tissues of HT plus PTC group, and 15 cases of RET/PTC rearrangement in cancer tissues of simple PTC group, with a proportion of 37.8% (28/74) and 19.7% (15/76), respectively, which was consistent with the existing statistical reports.
So far, there have been no significant reports of synergistic effects of RET/PTC rearrangements and BRAF mutations in the development of papillary thyroid carcinoma. However, it has been found that BRAF mutation, RET/PTC rearrangement and RAS mutation may play a role through RET/PTC-RAS-BRAF signal transduction pathway. Previous studies have confirmed that these three genetic events are independent of each other. Therefore, some scholars believe that BRAF mutation, RET rearrangement and RAS mutation will not appear in the same case. Any of the three genetic events in MAPK pathway is sufficient to promote the formation of thyroid cancer.20,21 Although they are all in the same signal transduction pathway, the steps they play a role may be different. However, some scholars believe that BRAF mutation and RET rearrangement can promote the occurrence of PTC.22 Some studies have found that BRAF mutation and RET/PTC rearrangement coexist in patients with recurrent papillary thyroid carcinoma.23 However, this situation has not been found in this group of studies.
Some scholars believe that HT can evolve into PTC, and then many studies believe that the relationship between HT and PTC is very close, but some scholars believe that the coexistence of HT and PTC is only accidental, and the mechanism of their coexistence is still not very clear.15 At present, it is generally believed that HT is one of the risk factors for the pathogenesis of PTC. Some scholars believe that HT, as an autoimmune disease, can cause PTC by mediating the recombination of RET/PTC gene and further transcriptional regulation.24,25 In this study, HT was positively correlated with positive RET gene rearrangement. Some scholars believe that the thyroid hormone secreted by HT is decreased due to low autoimmune function, and TSH is up-regulated by negative feedback, resulting in the continuous proliferation and differentiation of thyroid gland cells, which provides conditions for the growth of tumour cells, but there is still no agreement on the mechanism of HT participating in the progression of PTC.26
There were various limitations to the current study. First, the sample size is not large enough. Insufficient sample size affects the efficiency of the test, resulting in the inability to detect existing differences. Future research should aim to expand samples to enhance external validity. Secondly, the duration of this study was short and there was a lack of follow-up data. Therefore, the evidence for evaluating prognosis is not yet sufficient.
CONCLUSION
There is a certain correlation between HT and PTC. HT can promote the pathological changes of PTC, which may involve oncogene mutation and rearrangement, endocrine mechanism, immune mechanism, and so on. Similar immune targets and pathogenesis are some of the reasons for the high incidence of HT complicated with PTC. However, the mechanism and signal pathway of carcinogenesis at the molecular level remains to be answered, which depends on further research in the future. With a further understanding of the process of HT complicated with PTC, the detection of BRAF V600E mutation and RET/PTC may be helpful to early predict the transformation of HT to PTC and improve the early diagnosis rate of PTC, to guide clinical treatment and improve the prognosis of patients.
FUNDING:
This study was funded by a grant from the Applied Basic and Innovative Environment Research Project of Wuhu, China (2021jc2-2 to Min Xie).
ETHICAL APPROVAL:
The present study was carried out in accordance with the Declaration of Helsinki and approved by the Clinical Research Ethics Committee of East China Normal University affiliated Wuhu Hospital (Wuhu No.2 People’s Hospital) (approval no. 2021(31-46)).
PATIENTS’ CONSENT:
Written informed consents were taken from all patients included in the study.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
MX: Conception and design, drafting of the manuscript, and material preparation.
ZXXU: Data curation, formal analysis, writing review, editing.
MD: Supervision.
YYZ: Formal analysis, writing review and editing.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 2017; 317(13):1338-48. doi: 10.1001/ jama.2017.2719.
- Ehlers M, Schott M. Hashimoto's thyroiditis and papillary thyroid cancer: Are they immunologically linked? Trends Endocrinol Metab 2014; 25(12):656-64. doi: 10.1016/ j.tem. 2014.09.001.
- Moon S, Chung HS, Yu JM, Yoo HJ, Park JH, Kim DS, et al. Associations between hashimoto thyroiditis and clinical outcomes of papillary thyroid cancer: A meta-analysis of observational studies. Endocrinol Metab (Seoul) 2018; 33(4):473-84. doi: 10.3803/EnM.2018.33.4.473.
- Wang L, Li W, Ye H, Niu L. Impact of hashimotoas thyroiditis on clinicopathologic features of papillary thyroid carcinoma associated with infiltration of tumor-infiltrating lymphocytes. Int J Clin Exp Pathol 2018; 11(5):2768-75.
- Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto's thyroiditis: A meta-analysis. Eur J Endocrinol 2013; 168(3):343-9. doi: 10.1530/EJE-12-0903.
- Liang J, Zeng W, Fang F, Yu T, Zhao Y, Fan X, et al. Clinical analysis of hashimoto thyroiditis coexistent with papillary thyroid cancer in 1392 patients. Acta Otorhinolaryngol Ital 2017; 37(5):393-400. doi: 10.14639/0392-100X-1709.
- Anil C, Goksel S, Gursoy A. Hashimoto's thyroiditis is not associated with increased risk of thyroid cancer in patients with thyroid nodules: a single-center prospective study. Thyroid 2010; 20(6):601-6. doi: 10.1089/thy.2009.0450.
- Song E, Jeon MJ, Park S, Kim M, Oh HS, Song DE, et al. Influence of coexistent Hashimoto's thyroiditis on the extent of cervical lymph node dissection and prognosis in papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2018; 88(1): 123-28. doi: 10.1111/cen.13475.
- Guerra A, Zeppa P, Bifulco M, Vitale M. Concomitant BRAF(V600E) mutation and RET/PTC rearrangement is a frequent occurrence in papillary thyroid carcinoma. Thyroid 2014; 24(2):254-9. doi: 10.1089/thy.2013.0235.
- Chern GM, Wang M, Wang JF. Analysis of the clinic pathological features of patients with thyroid papillary carcinoma with hashimoto thyroiditis. J Tongji University (Medical Edition) 2018; 39(3):70-3.
- Xing BD, Zhao YH, Yu XL. Correlation analysis of clinicopathological features between Hashimoto's thyroiditis and thyroid papillary carcinoma. Modern Oncology Medicine 2020; 28(22):3873-7.
- Uhliarova B, Hajtman A. Hashimoto's thyroiditis - an independent risk factor for papillary carcinoma. Braz J Otorhinolaryngol 2018; 84(6):729-35. doi: 10.1016/j.bjorl. 2017.08.012.
- Scheffel RS, Dora JM, Maia AL. BRAF mutations in thyroid cancer. Curr Opin Oncol 2022; 34(1):9-18. doi: 10.1097/ CCO.0000000000000797.
- Kim SK, Woo JW, Lee JH, Park I, Choe JH, Kim JH, et al. Role of BRAF V600E mutation as an indicator of the extent of thyroidectomy and lymph node dissection in conventional papillary thyroid carcinoma. Surgery 2015; 158(6):1500-11. doi: 10.1016/j.surg.2015.05.016.
- Yan C, Huang M, Li X, Wang T, Ling R. Relationship between BRAF V600E and clinical features in papillary thyroid carcinoma. Endocr Connect 2019; 8(7):988-96. doi: 10. 1530/EC-19-0246.
- Sun J, Zhang J, Lu J, Gao J, Ren X, Teng L, et al. BRAF V600E and TERT promoter mutations in papillary thyroid carcinoma in Chinese patients. PLoS One 2016; 11(4):e0153319. doi: 10.1371/journal.pone.0153319.
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014; 159(3):676-90. doi: 10.1016/j.cell.2014.09.050.
- Romei C, Elisei R. RET/PTC Translocations and clinico-pathological features in human papillary thyroid carcinoma. Front Endocrinol (Lausanne) 2012; 3:54. doi: 10.3389/fendo. 2012.00054.
- Khan MS, Qadri Q, Makhdoomi MJ, Wani MA, Malik AA, Niyaz M, et al. RET/PTC gene rearrangements in thyroid carcino-genesis: Assessment and clinicopathological correlations. Pathol Oncol Res 2020; 26(1):507-13. doi: 10.1007/s12253- 018-0540-3.
- Xu X, Quiros RM, Gattuso P, Ain KB, Prinz RA. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res 2003; 63(15):4561-7.
- Silva JM, Rodriguez R, Garcia JM, Muñoz C, Silva J, Dominguez G, et al. Detection of epithelial tumour RNA in the plasma of colon cancer patients is associated with advanced stages and circulating tumour cells. Gut 2002; 50(4):530-4. doi: 10.1136/gut.50.4.530.
- Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol 2003; 120(1):71-7. doi: 10.1309/ND8D-9LAJ-TRCT-G6QD.
- Zhu Z, Ciampi R, Nikiforova MN, Gandhi M, Nikiforov YE. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: effects of the detection methods and genetic heterogeneity. J Clin Endocrinol Metab 2006; 91(9): 3603-10. doi: 10.1210/jc.2006-1006.
- Xun YP, Jiang YP, Fu JY. BRAF gene mutation in papillary thyroid carcinoma was detected by probe amplification block mutation method and digital PCR method. J Clinical Experimental Pathology 2021; 37(2):227-9.
- Dong S, Xie XJ, Xia Q, Wu YJ. Indicators of multifocality in papillary thyroid carcinoma concurrent with Hashimoto's thyroiditis. Am J Cancer Res 2019; 9(8):1786-95.
- Hanege FM, Tuysuz O, Celik S, Sakallıoglu O, Arslan Solmaz O. Hashimoto's thyroiditis in papillary thyroid carcinoma: a 22-year study. Acta Otorhinolaryngol Ital 2021; 41(2): 142-45. doi: 10.14639/0392-100X-N1081